Abstract
Background:
Obesity, a nationwide health issue, has related medical costs ranging between $147-210 billion per year in United States and has been associated with a 3.5-fold increased risk of developing NAFLD. In obesity, platelets work in a pleotropic manner with vascular and immune cells to amplify the chronic inflammatory process. Interestingly, studies have demonstrated that platelet numbers and reactivity are increased in obese individuals. The emerging role of activated platelets during obesity induced inflammation introduces the novel concept of platelet targeted therapeutic interventions.
Kopec et al, further supports the idea that the mechanism underlying the progression of obesity lies in a platelet mediated pro-inflammatory state, illustrating that there is extravascular fibrin(ogen) deposition, macrophages and inflammatory cytokines within white adipose tissue and liver of mice on western diet. Kopec et al uses a fibrinogen mutant mouse (Fiby390-396 ) which lacks the binding motif for Mac-1 and inhibits the ligand interaction with leukocytes, diminishing inflammation, reducing macrophage counts, reducing weight, protects mice from NAFLD and glucose dysmetabolism. Taken together, all evidence points towards a platelet/fibrinogen/leukocyte pathophysiological mechanism underlying the development of obesity.
TREM-Like Transcript-1 (TLT-1) is a platelet specific receptor found in the a-granules of platelets and released to the surface upon platelet activation. TLT-1 is a type 1 receptor that, like the integrin a2bb3, binds fibrinogen and facilitates platelet aggregation . However, although TLT-1 may assist in clot formation and hemostasis to arrest bleeding in a non-inflammatory/nonimmune mediated setting, TLT-1's main association is with regulating inflammatory-derived bleeding. This is demonstrated by increased hemorrhage after inflammatory treatments such as lipopolysaccharide LPS in the treml1 -/- mice as compared to controls.
Considering the emerging evidence in support of a platelet-fibrinogen receptor ligand interaction as a key mechanism underlying the development of obesity and that TLT-1, a platelet specific receptor binds fibrinogen and mediates leukocyte trafficking, our laboratory set out to determine whether TLT-1 could be implicated as the main culprit underlying this mechanism. When placed on a western diet, treml1 -/-mice are more prone to weight gain, based on these finding we hypothesize that: The TLT-1/Fibrinogen molecular interaction regulates metabolic inflammation in obesity
Aims:
Evaluate the effects of western diet on obesity and NAFLD in the treml1 -/- mouse model
Methods:
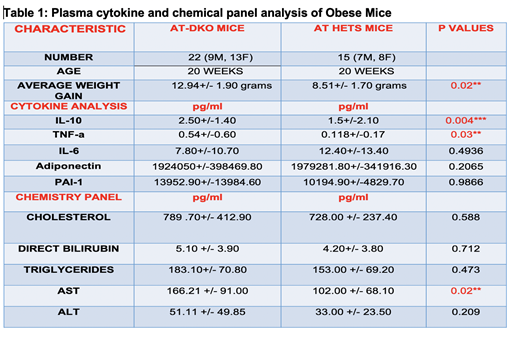
TLT-1 (treml1 -/-) - apolipoprotein E (apoe -/-) double null (AT-DKO;n=11) mice and control apoe +/-/treml1 +/- littermate controls (AT-Hets;n=20) were fed western diet for 20 weeks. Plasma samples were collected for adipokine, glucose, insulin, liver enzyme and lipid profiling. Mouse were perfused, liver and adipose tissue were collected for histological analysis.
Results:
Overall AT-DKO mice gained more weight compared to AT-Hets (12.94±1.90 vs 8.51±1.70 grams p=0.02). Plasma analysis demonstrates that the AT-DKO have higher levels of TNF-a (0.54±0.60 vs 0.118±0.17 pg/ml p=0.03), and IL-10 (2.50±1.40 vs 1.50±2.10 pg/ml p=0.004) compared to littermate controls. Histological analysis of livers illustrates increased lipid vacuoles and inflammatory foci in the AT-DKO mice as compared to controls, while preliminary data is not significant for these differences, liver damage in the AT-DKO was significantly greater as demonstrated by increased AST levels (166.21±91.00 vs 102±68.10 U/L p=0.02). Moreover, the AT-DKO mice had higher levels of ALT, direct bilirubin, cholesterol, pai-1 , triglycerides and lower IL-6 and Adiponectin (Table 1 data not significant). These findings suggest that in the absence of TLT-1 these mice are more prone to liver disfunction , hyperlipidemia and inflammatory alterations.
Conclusions:
Mutant AT-DKO mice are more prone to obesity and NAFLD compared to littermate controls, suggesting that TLT-1, a platelet gene, plays a surprising role in metabolism. Further investigation could adjudicate TLT-1 administration as a potential therapeutic intervention for prevention and amelioration of Obesity and related pathologies. The current state of this project will be reported here.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal